四环素在方解石上的吸附与解吸
来源:56doc.com 资料编号:5D28565 资料等级:★★★★★ %E8%B5%84%E6%96%99%E7%BC%96%E5%8F%B7%EF%BC%9A5D28565
资料以网页介绍的为准,下载后不会有水印.资料仅供学习参考之用. 密 保 惠 帮助
资料介绍
四环素在方解石上的吸附与解吸(论文7500字)
摘要:采用室内试验的方法,研究了四环素(TC)在方解石上的吸附与解吸,主要包括解吸动力学研究和3个不同pH下的等温吸附、解吸研究。结果表明:(1)四环素在方解石上的解吸较快,5h后便渐趋平衡;(2)3个pH条件下,四环素初始浓度的不同时都对Ca浓度和pH值影响甚微,说明四环素对原本溶液体系的稳定几乎没有影响;(3)在pH=7.7时,四环素在方解石上的等温吸附解吸既可以用Langmuir等温吸附方程拟合又可以用Freundlich等温吸附方程拟合,然而在pH=8.3和pH=9.1时,四环素在方解石上的吸附解吸结果只能用Freundlich等温吸附方程很好地拟合出来;(4)在3个pH条件下的等温吸附解吸的结果分析中得出,四环素吸附量随浓度的变化曲线与四环素解吸量随浓度的变化曲线十分相像且趋势一致,并且在用Langmuir和Freundlich模型拟合时,其能够较好模拟的,相关系数差距不大,可以推测四环素在方解石上的吸附行为是部分可逆的。
关键词:四环素;方解石;吸附;解吸
Adsorption and desorption of tetracycline in calcite
Abstract:Using laboratory test methods to study the tetracycline (TC) in the calcite adsorption and desorption, including adsorption isotherm and desorption kinetics of three different PH under desorption studies. The results showed that: (1) tetracycline desorption on calcite faster, 5h after gradually balance.(2) Under the conditions of pH 3, the initial concentration of tetracycline is not the same for both Ca concentration and pH have little effect, indicating almost no impact on the stability of tetracycline original solution system. (3) at pH = 7.7, the tetracycline in the calcite isothermal adsorption and desorption can be used both Langmuir isotherm equation fitting they can use Freundlich isotherm equation was, however, pH = 8.3 and pH = 9.1, the tetracycline in calcite results on adsorption and desorption can be well fitted out with Freundlich isotherm equation.(4) at three pH conditions of isothermal adsorption and desorption analysis of the results obtained, tetracycline adsorption increases with the concentration curve and tetracycline desorption increases with the concentration curve is very similar and consistent trend, and intends to use Langmuir and Freundlich models timeliness, which can better simulate the correlation coefficients did not differ much, you can speculate on the adsorption behavior of tetracycline in calcite is partially reversible.
Key words: tetracycline、calcite、adsorption、desorption
目 录
1 引言 5
1.1研究背景 5
1.2 国内外研究现状 5
1.3 主要研究内容及目的 5
2 实验材料与方法 6
2.1实验材料 6
2.1.1实验试剂 6
2.1.2 实验器材 6
2.2 实验方法及步骤 7
2.2.1 预平衡 7
2.2.2解吸动力学实验 7
2.2.3等温吸附解吸实验 7
2.2.4 实验分析方法 8
2.2.4.1 测试方法 8
2.2.4.2实验数据分析 8
3 结果与讨论 8
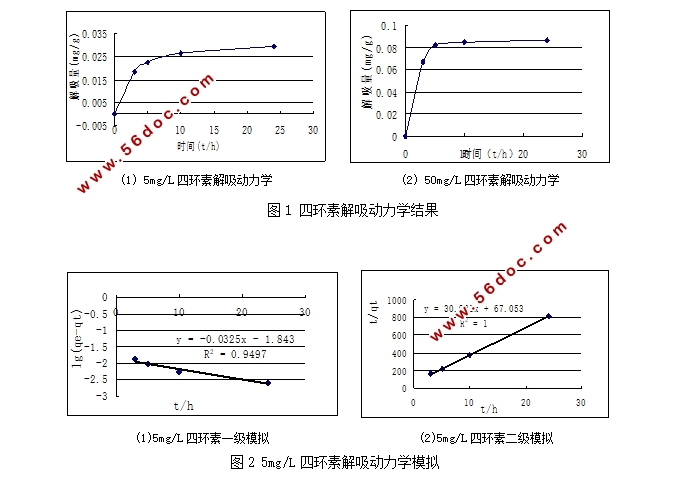
3.1 解吸动力学实验 8
3.2 等温吸附解吸实验 10
3.2.1 pH=7.7时的吸附解吸 11
3.2.2 pH=8.3时的吸附解吸 12
3.2.3 pH=9.1时的吸附解吸 14
4 结论 16
参考文献 18
致谢 19
|



