注射用阿奇霉素生产车间设计
来源:56doc.com 资料编号:5D4483 资料等级:★★★★★ %E8%B5%84%E6%96%99%E7%BC%96%E5%8F%B7%EF%BC%9A5D4483
资料以网页介绍的为准,下载后不会有水印.资料仅供学习参考之用. 密 保 惠 帮助
资料介绍
摘 要: 本设计通过对注射用阿奇霉素生产工艺的研究,按照GMP对制药行业洁净区的设计要求,设计了一套注射用阿奇霉素的生产车间,该车间平面布局包括百级,万级,十万级,三十万级四个净化区间。设计确定阿奇霉素工艺流程,设备选型,计算了原辅料消耗,规范了生产工艺操作程序。该车间年生产能力达4000万支阿奇霉素。
关键词:GMP;注射用阿奇霉素;洁净区;车间设计
Workshop dedign of Azitromycin for injection
Abstract: After studying the manufacturing technique of the Azithromycin for injection, the author of this paper has designed a Azithromycin for injection production workshop accroding to the requirements for the pHarmaceutical industry’s purification areas given by Good Manufacturing Practice(GMP). The plane figure of this workshop includes purification areas in four grades, which are the hundred grades, ten thousand grades, one hundred thousand grades and three hundred thousand grades. The design has showed the process flow of Azithromycin and the lectotype selection. It has also worked out the consumption of supplementary material and has standarded the operational procedure of the manufacturing technique. The workshop designed in the paper has a yearly productive capacity of 40 million ones.
Key words:GMP; Azithromycin for Injection; Clean area; Workshop design
目 录 11000字
摘要 1
关键词 1
1 前言 2
2 项目总论 2
2.1 项目背景 2
2.2 项目投资概况 3
2.3 工作制度及定员 3
2.4 质量标准 4
3 市场分析 5
3.1 行业发展情况 5
3.2 项目产品市场分析 5
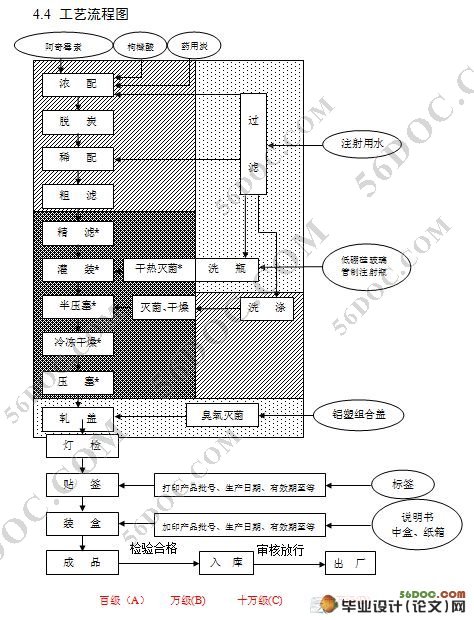
4 工艺流程设计 6
4.1 适用范围 6
4.2 产品信息 6
4.3 产品处方 6
4.4 工艺流程图 7
4.5 生产操作及工艺条件 8
5 物料平衡与经济技术指标 13
5.1 物料平衡 13
5.2 经济技术指标 14
6 项目建设与设备选型 15
6.1 项目建设外部条件及车间平面布局 15
6.2 主要生产设备及关键计量器具选型 18
7 技术安全及劳动保护 20
7.1 技术安全 20
7.2 劳动保护 21
参考文献 21
致谢 22
|



